Short Communication
Grodno State Medical University, Gorkogo St., Grodno, Republic of Belarus.
*Corresponding Author: Elizaveta I Bon
Citation: Elizaveta I Bon, N. Ye. Maksimovich, S. M. Zimatkin, O.B. Ostrovskaya, V. Yu. Smirnov, N. V. Kokhan, Grodno State Medical University, Gorkogo St., Grodno, Republic of Belarus. Characterization Of Changes In The Ultrastructure Of Neurons In The Cerebral Cortex Of
Rats With Subtotal Cerebral Ischemia Against The Background Of The Introduction Of Omega-3 polyunsaturated Fatty Acids, Journal of Clinical and Medical Case Reports and Reviews. 2(3).
Copyright: © 2022 Elizaveta I Bon, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited..
Received: December 05, 2022 | Accepted: December 16, 2022 | Published: December 26, 2022
Abstract
The aim of this study was to elucidate the ultrastructure of neurons in the parietal cortex and hippocampus of rats with subtotal cerebral ischemia against the background of the introduction of omega-3 polyunsaturated fatty acids.The neurons of the hippocampus, as a phylogenetically older part of the cerebral cortex, are less sensitive to hypoxia, which may be the reason for the therapeutic effect of Omega3 polyunsaturated fatty acids.Thus, the administration of ω-3 polyunsaturated fatty acids has a corrective effect on the ultrastructure of hippocampal neurons under conditions of subtotal cerebral ischemia, which manifested itself in an increase in the density of mitochondrial cristae and a decrease in the density of lysosomes.
Keywords: cerebral ischemia, neurons, polyunsaturated fatty acids
Introduction
With cerebral ischemia, a chain of pathogenetic disorders develops in its structures, among which one of the leading ones is energy deficiency, which leads to the development of cellular pathology due to violations of homeostasis, enzyme activity, membrane integrity and energy pumps [3,4,14]. Under conditions of cerebral ischemia, synaptic transmission mechanisms are selectively disrupted, which contributes to disruption of autoregulation of local blood flow, the development of vasospasm, increased platelet aggregation and the development of intravascular stasis, aggravating hypoxia and increasing energy deficiency [8,12,15]. The work of enzymes, including sodium-potassium ATPase, is disrupted, leading to an imbalance of ions and cerebral edema [5,6,7].
As you know, Omega-3 polyunsaturated fatty acids ensure the functioning of cell membranes, transmembrane ion channels, participate in the regulation of physiological processes and the implementation of the main functions of neurons – the transmission of impulses and the operation of receptors [1,2,13]. Brain neurons are electrically active cells rich in ion channels, and therefore can be sensitive to their deficiency [9,10,11].
It was supposed to study changes in the ultrastructure of neurons in the parietal cortex and hippocampus of rats with subtotal cerebral ischemia on the background of the introduction of omega-3 polyunsaturated fatty acids.
Materials and methods of research
The experiments were carried out on 18 outbred white rats weighing 260±20 g in compliance with the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010 on the protection of animals used for scientific purposes.
Modeling of cerebral ischemia was carried out under conditions of intravenous thiopental anesthesia (40-50 mg/kg).
Subtotal cerebral ischemia was modeled by simultaneous ligation of both common carotid arteries. The material was taken 1 hour after the operation.
To study the effects of ω-3 polyunsaturated fatty acids, animals before cerebral ischemia were intragastrically injected with ω-3 polyunsaturated fatty acids at a dose of 5 g/kg of body weight for a week. The control group consisted of sham-operated rats of similar sex and weight.
Electron microscopic studies were performed in the parietal cortex and hippocampus of the brain of rats
Immediately after decapitation and quick extraction of the brain, sections of the parietal cortex and hippocampus were cut out with a blade and placed in 1% osmium fixative in Millonig's buffer (pH=7.4) for 2 hours at 4°C.
Next, the sections were washed in a mixture of Millonig's buffer (20 ml) and sucrose (900 mg), dehydrated in alcohols of increasing concentration, a mixture of alcohol and acetone, pure acetone; passed through a mixture of resins (araldite M + araldite H + dibutyl phthalate + DMR-30) and acetone and enclosed in a mixture of resins.
Semi-thin sections (about 350 nm thick) were made on an MT-7000 ultramicrotome (RMC, USA), stained with methylene blue, and sections of the inner pyramidal layer of the parietal cortex and the pyramidal layer of the field CA1 of the hippocampus necessary for study were cut out with a blade.
Ultrathin sections (about 35 nm thick) were made on the same ultramicrotome, assembled on support grids, and counterstained with uranium acetate and lead citrate. To do this, the meshes with sections were dipped into a drop of uranyl acetate and kept for 20 minutes in the dark at room temperature, then washed in 3 portions of bidistilled water for 5 seconds and contrasted with lead citrate for 8 minutes, washed in 3 portions of bidistilled water for 5 seconds.
The resulting preparations were studied under a JEM-1011 electron microscope (JEOL, Japan) and photographed with an Olympus MegaView III digital camera (Olympus Soft Imaging Solutions, Germany).
Morphometry of ultrastructures was performed using the Image Warp image processing program (Bit Flow, USA), for which mitochondria, the Golgi complex, the granular endoplasmic reticulum, ribosomes, and lysosomes were circled on a computer monitor. Density, size, shape of organelles, density and length of mitochondrial cristae, sizes of lysosomes and their density, the number of endoplasmic reticulum-bound and free ribosomes were measured, and their ratio was calculated.
To prevent a systematic measurement error, brain samples from the compared control and experimental groups of animals were studied under the same conditions.
As a result of the research, quantitative continuous data were obtained. Since the experiment used small samples that had a non-normal distribution, the analysis was performed by nonparametric statistics using the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). The data are presented as Me (LQ; UQ), where Me is the median, LQ is the value of the lower quartile; UQ is the value of the upper quartile. Differences between groups were considered significant at p<0>
When conducting an ultrastructural study of brain neurons in rats with subtotal cerebral ischemia lasting 1 hour against the background of the introduction of the drug ω-3 polyunsaturated fatty acids "Omegamed" at a dose of 5 g/kg of body weight during the week, no changes were detected in the parietal cortex. The parameters of ultramicroscopic morphometry of organelles of parietal cortex neurons did not differ (p>0.05), figures 1, 2, table 1.
Table 1. Parameters of ultramicroscopic morphometry of neuronal organelles in the parietal cortex and hippocampus of rats with subtotal cerebral ischemia and against the background of the introduction of omega-3 polyunsaturated fatty acids, Me(LQ;UQ).
| Index | parietal cortex | hippocampus | |||||
| Control | SCI 1 hour | SCI+Omega-3 | Control | SCI 1 hour | SCI+Omega-3 | ||
| Mitochondria | density | 1,8(1,7;2,2) | 1,6(1,5;1,7) | 1,7(1,6;1,7) | 2,1(1,7;2,2) | 1,8(1,6;1,9) | 2,0(1,6;2,3) |
| area, µm2 | 0,26 (0,17;0,37) | 0,18(0,14;0,20) * | 0,22(0,17;0,22) | 0,21(0,17;0,26) | 0,14(0,13;0,22) * | 0,15(0,10;0,20) | |
| form factor, unit | 0,63(0,61;0,72) | 0,89(0,83;0,94) | 0,88(0,78;0,91) | 0,71(0,60;0,75)* | 0,88(0,81;0,94)* | 0,82(0,80;0,88) | |
| elongation factor, units | 3,8(3,5;4,1) | 1,8(1,7;2,1) | 1,8(1,7;1,9) | 2,1(1,9;2,5) | 1,6(1,4;1,8)* | 1,7(1,6;1,8)* | |
| mitochondrial crist density | 76(71;82) | 44(37;49)* | 40 (36;50) | 62(59;72) | 57(52;60)* | 67(60;73)* | |
| length of mitochondrial cristae / µm2 | 12(10;15) | 6(4;7)* | 5(4;7) | 13(12;18) | 8(6;9)* | 10(7;12) | |
| Ribosomes | amount /µm2 | 20,9(19,3;22,7) | 21,9(18,9;22,8)* | 20,5(19,3;22,0)* | 20,0(18,1;22,8) | 20,6(17,8;23,7)* | 19,5(17,6;21,6)* |
| free / µm2 | 4,7(4,1;5,8) | 18,3(15,4;18,9)* | 17,0(16,2;17,8)* | 6,0(4,8;7,3) | 15,3(14,3;17,4)* | 14,5(13,3;15,8) | |
| bound /µm2 | 16,2(15,2;16,9) | 3,6(3,5;3,9)* | 3,5(3,1;4,2)* | 14,0(13,3;15,5) | 5,3(3,5;6,3)* | 5,0(4,3;5,8)* | |
| ratio of bound and free ribosomes | 3,4 | 0,2 | 0,2 | 2,3 | 0,3 | 0,3 | |
Lysosomes
| density | 0,4(0,3;0,5) | 1,7(1,6;1,9) | 1,6(1,5;1,8) | 0,5(0,4;0,6) | 1,5(1,4;1,6) | 1,2(1,1;1,3) + |
| area, µm2 | 0,02(0,01;0,03) | 0,32(0,19;0,48) | 0,21(0,17;0,34) | 0,03(0,02;0,04) | 0,24(0,17;0,32) | 0,08(0,07;0,09) + | |
Note: numerical values are presented as methochondria (LQ; UQ), * - p < 0>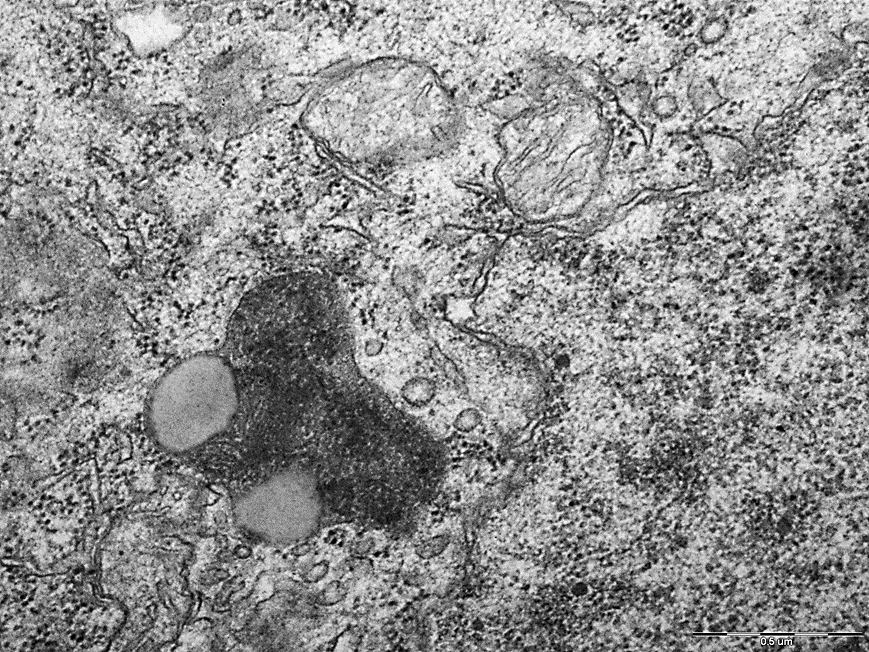
Figure 1. Mitochondria and lysosomes of neurons in the parietal cortex of rats of the subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids group. N – nucleus, Nm – nuclear membrane, Mt – mitochondria, L – lysosomes. Magnification: 50000. Electron diffraction pattern.
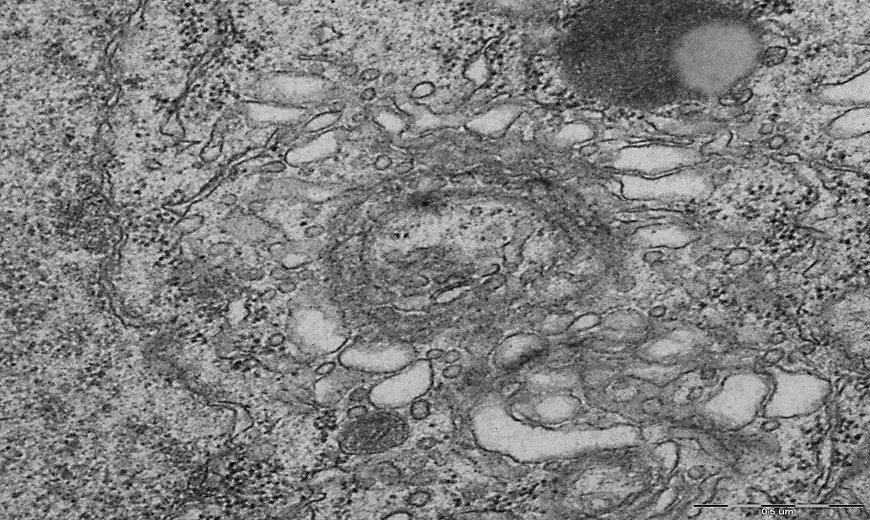
Figure 2. The predominance of free ribosomes, deformation and expansion of the cisterns of the endoplasmic reticulum and the Golgi complex in the cytoplasm of neurons of the parietal cortex of rats of the group "subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids". N – nucleus, Nm – nuclear membrane, FR – free ribosomes, GC – Golgi complex. Magnification: 40000. Electron diffraction pattern.
Compared to the indicators in the control group, in the subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids group, in the parietal cortex, the mitochondrial elongation factor was less by 53(47;57)%, p<0>
Along with changes in mitochondria, an increase in the number of free ribosomes in the cytoplasm of TC neurons was noted in rats of the subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids group, compared with the indicators in the control group, by 72 (65; 78)%, p<0>
The density of lysosomes in the cytoplasm of neurons of the parietal cortex increased by 75(69;78)%, p<0>0.05).
Free ribosomes also predominated in the cytoplasm of neurons in Hp rats of the subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids group, amounting to 14.5 (13.7; 16.1) per unit area. Disorganization of the cisterns of the endoplasmic reticulum and the Golgi complex was observed, however, in rats of the subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids group, compared to the subtotal cerebral ischemia group, the number and size of lysosomes decreased by 20 (16; 27)% , p<0>
Figure 3. Mitochondria, the predominance of free ribosomes, the expansion of cisterns of the endoplasmic reticulum and the Golgi complex of the cytoplasm of neurons in the CA1 field of the hippocampus of rats "subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids". N – nucleus, Nm – nuclear envelope, Mt – mitochondria, L – lysosomes, GER – granular endoplasmic reticulum, FR – free ribosomes, GC – Golgi complex. Magnification: 50000. Electron diffraction pattern.
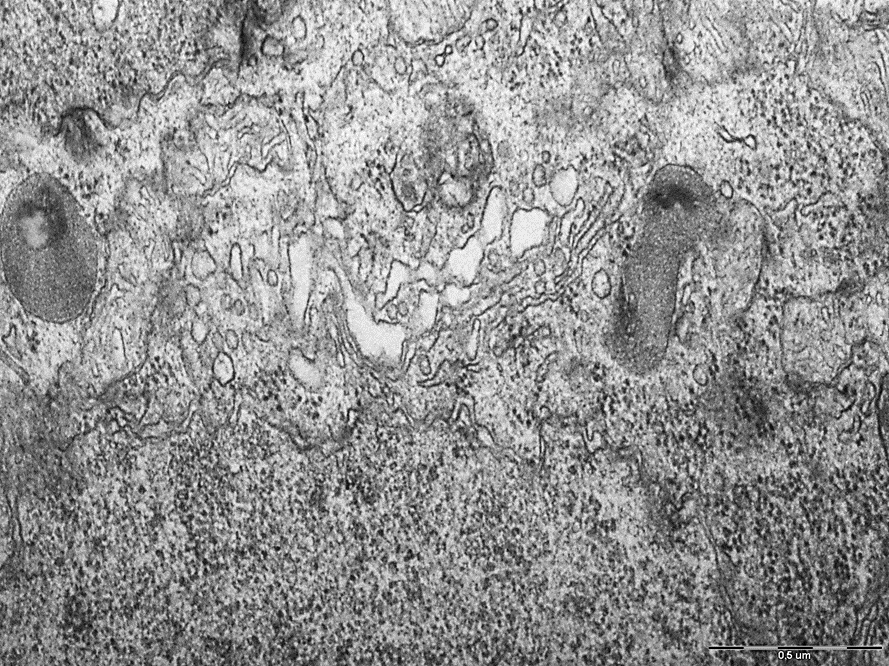
Figure 4. Lysosomes and the Golgi complex of neurons in the CA1 field of the rat hippocampus "Subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids". N – nucleus, Nm – nuclear membrane, L – lysosomes, GC – Golgi complex. Magnification: 50000. Electron diffraction pattern.
However, there was no complete correction of ultrastructural changes. So, compared with the indicators in the "control" group, in the "subtotal cerebral ischemia + Omega-3 polyunsaturated fatty acids" group, the mitochondrial elongation factor remained 19 (13; 23)% less, p<0>
The beneficial effect of polyunsaturated fatty acids (Omega-3 polyunsaturated fatty acids) on the state of neurons in the cerebral cortex under conditions of subtotal cerebral ischemia may be due to an improvement in the rheological properties of blood due to a decrease in the production of thromboxane A by platelets and an increase in the level of tissue plasminogen activator and a decrease in blood viscosity [9,10]. Omega-3 polyunsaturated fatty acids also have anti-inflammatory effects due to their incorporation into the phospholipid layer of cell membranes. In addition, polyunsaturated fatty acids, affecting the synthesis of prostaglandins, regulate vascular tone and prevent vascular vasoconstriction under the influence of catecholamines, which causes a moderate vasodilatory effect [11,13].
Omega-3 polyunsaturated fatty acids are also able to reduce the activity of oxidative stress, as they are a necessary component in the recycling of the main endogenous antioxidants. They also have their own antioxidant and lipotropic activity due to the activation of the formation of coenzyme A, the transport of acetate and fatty acids from the cytosol to the mitochondrial matrix, and the stabilization of cell membranes [1,2,10].
The neurons of the hippocampus, as a phylogenetically older part of the cerebral cortex, are less sensitive to hypoxia, which may be the reason for the therapeutic effect of Omega-3 polyunsaturated fatty acids.
Thus, the administration of ω-3 polyunsaturated fatty acids has a corrective effect on the ultrastructure of hippocampal neurons under conditions of subtotal cerebral ischemia, which manifested itself in an increase in the density of mitochondrial cristae and a decrease in the density of lysosomes.
References
- Bon L.I., Maksimovich N.E., Zimatkin S.M. Morphology of rat brain neurons in subtotal ischaemia and introduction of L-NAME and omega-3 polyunsaturated fatty acids // Journal of Medical Science. – 2020. – Р. 1–8.
Publisher | Google Scholor - Bon L.I., Maksimovich N.Yе. Vasoprotective Effects of Omega-3 Polyunsaturated Fatty Acids in Cerebral Ischemia // International Journal of Cardiology and Cardiovascular Disorder. – 2021. – V. 2. – P. 1–5.
Publisher | Google Scholor - Clemens J.A. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants // Free Radic. Biol. Med. – 2000. – V. 28. – P. 1526–1531.
Publisher | Google Scholor - Colbourne F., Sutherland G.R., Auer R.N. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia // J. Neurosci. – 1999. – V. 19. – P. 4200–4210.
Publisher | Google Scholor - Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view // Trends Neurosci. – 1999. – V. 22. – P. 391–397.
Publisher | Google Scholor - Garcia J.H., Kamijyo Y. Cerebral infarction: evolution of histopathological changes after occlusion of a middle cerebral artery in primates // J. Neuropathol Expl Neurol. –1974. –V. 33. – P. 408–421.
Publisher | Google Scholor - Histologic assessment of neurons in rat models of cerebral ischemia / A. Eke [et al.] // J. Stroke. – 1990. – V. 21. – P. 299–304.
Publisher | Google Scholor - Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats / J.H. Garcia [et al.] // J. Stroke. – 1997. –– V. 28. – P. 2303–2309.
Publisher | Google Scholor - Kaliannan K., Li X. Y., Wang B. Multiomic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease // Commun Biology. – 2019. – V. 2, N 1. – P. 276–280.
Publisher | Google Scholor - Khunt D., Shrivas M., Polaka S. Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: a Comparative Study // Pharmacology Sciencific Technology. – 2020. – V. 21, N 2. – P. 45–50.
Publisher | Google Scholor - Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease / K. Kaliannan [et al.] // Commun Biology. – 2019. – V. 1. – P. 276–280.
Publisher | Google Scholor - Neuronal ischemic injury: light microscopy, ultrastructure and biochemistry / J.H. Garcia [et al.] // J. Acta Neuropathol (Berl). – 1978. – V. 43. – P. 85–95.
Publisher | Google Scholor - Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: a Comparative Study / D. Khunt [et al.] // Pharmacology Sciencific Technology. – 2020. – V. 21. – P. 45–50.
Publisher | Google Scholor - Snider B.J., Gottron F.J., Choi D.W. Apoptosis and necrosis in cerebrovascular disease // Ann N Y Acad Sci. – 1999. – V. 893. P. 243–253.
Publisher | Google Scholor - The heterogeneous temporal evolution of focal ischemic neuronal damage in the rat / M.O. Dereski [et al.] // J. Acta Neuropathol (Berl). – 1993. – V. 85. – P. 327–333.
Publisher | Google Scholor - The ultrastructure of “brain death,” II: electron microscopy of feline cortex after complete ischemia / H. Kalimo [et al.] // J. Virchows Arch B Cell Pathol. – 1977. – V. 25. – P. 207–220.
Publisher | Google Scholor
 Alcrut
Alcrut