Short Communication
- Former Director Grade Scientist, Centre for Cellular and Molecular Biology, Hyderabad, India.
- Former, Associate Professor, S. D. N. B. Vaishnav College for Women, Chennai, India.
*Corresponding Author: P D Gupta
Citation: P D Gupta, K Pushkala, FMT as an effective therapeutic agent for Endometriosis, Journal of Clinical and Medical Case Reports and Reviews V(2)I(2).
Copyright: © 2022 P D Gupta, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 10, 2022 | Accepted: July 25, 2022 | Published: July 30, 2022
Abstract
Many of the pathologies which were hither to like endometriosis couldn’t be solved by even by specific drugs or surgeries are now can be managed by balancing the composition of gut microbiota. Healthy man/woman faecal microbiota recently was tried to treat many patients suffering with various diseases including endometriosis and even to boost immune responses in adults and children. Future studies into the functional profile of the microbiome would greatly assist in the development of microbiome-based therapies to alleviate endometriosis symptoms and improve the quality of life of women suffering from.
Keywords: therapeutic agent; endometriosis; uterine
Introduction
The tissue similar to the endometrial, the inner lining of the uterus, when it grows abnormally on extra-uterine locations such as, ovaries, fallopian tubes, vagina, peritoneum, bladder and ureters, intestines, rectum, diaphragm and the pelvis was named as endometriosis. This painful disorder was known to gynaecologist even over 300 years ago. Pathology of endometriosis was unknown until the involvement of estrogen, cytokines, growth factors and initial inflammatory compounds such as lipopolysaccharides (LPS) which are important outer membrane components of gram-negative bacteria mediators were known in the growth of endometriosis [1]. According to a recent survey, 196 million women between the ages of 12 and 52 suffering with endometriosis affecting approximately 6 to 10% of reproductive-age women globally [2]. Endometriosis occurs in approximately 2–5% of postmenopausal women with more convoluted patho-physiology than the premenopausal subjects [3].
The endometrial-like tissue on organs as mentioned above normally thickens, breaks down and bleeds with each menstrual cycle and due to lack of way to exit the body, gets trapped inside the body. When such type of growth takes place in the ovaries they are identified as Endometriomas (Fig. 1).
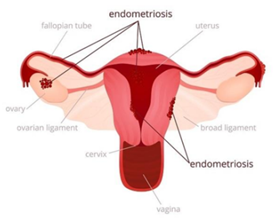
Fig. 1 Growth of endometrial tissue outside the uterus
Subjects with endometriosis suffers with health problems such as painful periods (dysmenorrhea), pain during intercourse, pain during bowel movements or urination, excessive bleeding, infertility, fatigue, diarrhoea, constipation, bloating or nausea especially during menstrual periods, dyspareunia, hematuria, dysuria, and dyschezia. In addition to the symptoms mentioned above, few may also suffer with pain in the catamenial pneumothorax, cyclical cough, and cyclical scar edema [3].
Retrograde menstruation, transformation of peritoneal cells, embryonic cell transformation, surgical scar implantation, endometrial cell transport and immune system disorder are suspected to be the causal factor for endometriosis. Even after a long 300 years, most of the literature still claims that the pathogenesis and/or pathophysiology of endometriosis are unclear. Studies have shown that the growth and progression of endometriosis continue even in an ovariectomized animal giving a clue that besides ovarian steroid hormones, the growth of endometriosis can be regulated by the innate immune system in the pelvic environment [1]. Degraded endometrial tissue during menstruation gets backflow, and subsequent attachment or invasion into the pelvis results in a physical or chemical tissue stress reaction in patients suffering with endometriosis [1;4;5].
Endometriosis can imitate other conditions with pelvic pain, such as pelvic inflammatory disease (PID) or ovarian cysts. Diagnosis becomes difficult and confused with irritable bowel syndrome (IBS), where bouts of diarrhoea, constipation and abdominal cramping could be observed. Situation becomes more complicated when IBS exists with endometriosis in therapeutics [6].
Immune–endocrine cross-talk between estrogen and bacterial endotoxin, a potential inflammatory mediator in the pelvic environment, could be involved as an additive inflammatory response and growth of endometriosis since, estrogen is also capable of inducing a pro-inflammatory response and endometriosis is a hormone dependent disease. Authors have a strong claim from series of studies that bacterial contamination in menstrual blood could also be the effect of endometriosis as well as capable of inducing the development of endometriosis since, they demonstrated that LPS regulates the expression of HGF and its receptor, c-Met, in the PF, ESCs, and EECs [1].
Management of Endometriosis
Differential treatment pattern is in vogue since, not all treatments work well for all women with endometriosis. Therapeutically analgesics such as non-steroidal anti-inflammatory drugs and hormonal treatment, such as low-dose combination oral contraceptives such as ethyl estradiol and progestins is in practice initially for every subject [3] followed by surgical excision of lesions, non-drug therapies, hormonal and non-hormonal therapies, or any of those approaches in combination depending on the situation.
Non-drug therapies focus on dietary intervention, physical therapy, and psychological intervention. Dietary interventions have been proved to have a satisfactory positive impact on endometriosis. For example, omega-3 polyunsaturated fatty acids (o-PUFAs) were found to lower patients’ pain scores [3;7]. Complete cure from Endometriosis is difficult since symptoms may return after the treatment is stopped or, in the case of surgery, as more time passes after the procedure [7].
Role of microbiota in the prognosis of endometriosis
Researchers are surprised to discover that the microbiome and endometriosis development are bidirectionally related, implying that any change in the host’s microbiome can have a significant impact on the development and progression of endometriosis [8]. Levels of bacterial colonization in the endometrial tissues and menstrual blood also was found to be higher in subjects suffering with the disease than women from the general population [3].
Christ et al. [9] and Medina-Perucha et al. [10] reported that women aged 35–44 years old suffer highest incidence of endometriosis. Additionally, Yamamoto et al. [11] were interested to understand if ethnicity/race is also contributory factor for the prevalence of endometriosis among IVF patients, and observed that a significantly higher prevalence among Asian women compared to Caucasians (15.7 vs. 5.8%, p < 0>
Emerging evidences suggest that there may be urogenital-gastrointestinal crosstalk exists resulting in the progression of the disease. Based on the availabe literature, some levels of changes in microbial signatures present among endometriosis patients, though these changes were not only restricted to the female reproductive tract but also at other sites including the gut as well as the peritoneal region [4].
Significance of Faecal Microbiota
“Bacterial contamination hypothesis” was proposed in endometriosis based on the study findings of intrauterine microbial colonization (IUMC) in women with endometriosis. This paved way for a new therapeutic potential in addition to the conventional estrogen-suppressing agent [1].
Yuan and the team in 2018 [12] reported predominance of Firmicutes and Actinobacteria phylum in mice with endometriosis induced via intra-peritoneal injection of endometrial segments, along with an elevated level of Bifidobactericeae and Alcaligenceae [12]. Consistent findings were also reported by Ni and the team in 2021 [13] where they discovered an increased abundance of Clostridium_sensu_stricto_1, Bifidobacterium, and Candidatus_Saccharimonas, on top of elevated Lactobacillus abundance in the gut microbiome of endometriosis in mice. Similar to findings from the rodents’ model the team [13] reported dynamic changes in the gut microbiome of olive baboons during their15-months study. For instance, the abundance of genera Succinivibrio, Prevotella, Megasphaera, Lactobacillus, and CF231 was decreased in faecal samples at three months post-induction of endometriosis; out of these genera, three of them—Succinivibrio, Prevotella, and CF231 abundance increased throughout the disease progression from six to nine months post-induction. An in-depth correlation study on microbial species and peripheral immune cells after induction of endometriosis identified that certain microbial populations in the GI tract displayed a positive correlation with immune cell populations (i.e., natural T regulatory cells and T helper 17 cells) at different study time points, but not the peritoneal and vaginal microbiome [3].
Another essential point to consider in the crosstalk of microbiota and host estrogen is estrogen-microbiome axis. The relationship between the microbiome and host immune response has been observed that imply that alterations in the estrobolome brought upon by dysbiosis can trigger estrogen-mediated pathologies, including endometriosis as well as endometrial cancer [3].
In-depth knowledge in this field further strengthen the very reason to take advantage of the microbiomes for treatment targets, since they have a potential in modulating the host immune system and alleviating pelvic inflammation in patients [3].
Mechanism of Action of the Gut Microbiota
The role of bacterial endotoxin (LPS) and Toll-like receptor 4 (TLR4) in endometriosis were studied the possible source of endotoxin in the pelvic environment was examined. Literature survey shows that LPS regulates the pro-inflammatory response in the pelvis and growth of endometriosis via the LPS/TLR4 cascade. The menstrual blood Escherichea coli contamination as well as endometrial samples are colonized with other microbes. A cross-talk between inflammation and ovarian steroids or the stress reaction also was observed in the pelvis. Treatment with GnRHa further worsens intrauterine microbial colonization, with the consequent occurrence of endometritis in women with endometriosis. In addition to the regulation the pro-inflammatory response LPS was found to induce and regulate the expression of cell–cell adhesion molecules (intercellular adhesion molecule 1/vascular cell adhesion molecule 1/fibronectin/laminin) and their receptors (integrin α3 and integrin α6) in the endometrium and pelvic peritoneum. In addition, hepatocyte growth factor (HGF) and fibronectin and laminin present in the endometrial and mesothelial cells could suggest that, probably after bacterial contamination, as an initial inflammatory mediator, LPS have an indirect effect on the development and progression of endometriosis, either alone or in combination with ovarian steroids or a tissue stress reaction in the pelvis. Further studies are required to strengthen this study's proposed concept in the management of endometriosis [1].
Recent, studies have shown that FMT can be used for the treatment of female reproductive tract diseases Due to the role of gut microbiota in the development of endometriosis, FMT could be an innovative treatment option for the treatment of endometriosis [14].
References
- Khan K N. et al.,( 2018) Bacterial contamination hypothesis: a new concept in endometriosis Reprod Med Biol. ; 17(2): 125–133.
Publisher | Google Scholor - https://emedicine.medscape.com › 254169-treatment. Endometritis Treatment & Management - Medscape
--> - Hooi-Leng Ser,et al. (2023) Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review Microorganisms. ; 11(2): 360.
Publisher | Google Scholor - https://my.clevelandclinic.org › 10857-endometriosis.27-Jul-2022 Endometriosis: Causes, Symptoms, Diagnosis & Treatment Cleveland Clinic
--> - https://emedicine.medscape.com ›Endometritis Treatment & Management - Medscape Reference 254169-treatment
--> - https://www.mayoclinic.org › syc-20354656 Endometriosis - Symptoms and causes Mayo Clinic
--> - https://www.nichd.nih.gov › conditioninfo › treatmen. What are the treatments for endometriosis? | NICHD
--> - Jiang I. et al. (2021) Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci; 22:5644.
Publisher | Google Scholor - Christ J.P. (2021), et al.Incidence, prevalence, and trends in endometriosis diagnosis: A United States population-based study from 2006 to 2015. Am. J. Obs. Gynecol.; 225:500. e1.
Publisher | Google Scholor - Medina-Perucha L., (2022) et al. Endometriosis prevalence and incidence trends in a large population-based study in Catalonia (Spain) from 2009 to 2018. Womens Health; 18:17455057221130566.
Publisher | Google Scholor - Yamamoto A. (2017) et al. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J. Assist. Reprod. Genet; 34:765–774.
Publisher | Google Scholor - Yuan, M. (2018) et al Endometriosis induces gut microbiota alterations in mice. Hum. Reprod, 33, 607–616.
Publisher | Google Scholor - Ni, Z. (2021) et al. Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. Am. J. Reprod. Immunol. , 86, e13471.
Publisher | Google Scholor - Quaranta G, (2019) et al. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 10:2653.
Publisher | Google Scholor
 Alcrut
Alcrut