Case Series
1 PBM Healing International, Hong Kong
2 Laser Research Centre,Faculty of HealthSciences, University of Johannesburg, Doornfontein, South Africa
*Corresponding Author: Dr. Alan Kwong Hing, PBM Healing International, Hong Kong.
Citation: Dr. Alan Kwong Hing and Dr. Michael R. Hamblin, Photobiomodulation for Primary Dysmenorrhea: A Retrospective Observational Case Series in Three Women Using a Dual-Wavelength FEM Belt and Proposal for a Pilot Clinical Trial, J. Women Health Care Res and Rep, V (4)I(4), DOI: 10.59468/2837-2166/110
Copyright: 2025 Alan Kwong Hing. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 09, 2025 | Accepted: December 13, 2025 | Published: December 10, 2025
Abstract
Objective:
We describe the PBM FEM belt, a photobiomodulation (PBM) device using 635-nm red and 880-nm near-infrared LEDs, along with preliminary clinicaldata for 3 cases regarding its application in the treatment of primary dysmenorrhea.
Methods:
We conducteda retrospective observational case series involvingthree women who utilized the FEM belt.Standardized case reportforms collected VisualAnalog Scale (VAS 0–10) pain scores, duration of relief, medication usage, satisfaction levels, and the likelihood of recommending the device. All three available cases during the review period were included; no cases were excluded.
Results:
The initial baseline VAS for all three women was 10. After one day of device usage, their VAS scores decreased by an average of 90% to a mean of 1. Two participants experienced immediate and rapid relief, and all three reported improvements on the initialday of use. Analgesic medication use was reducedor eliminated completely. In every case, satisfaction was 5 out of 5, and recommendation likelihood was rated 10 out of 10.
Conclusions:
The PBM FEM belt provided quick,non-invasive and immediate dysmenorrhea relief and reduced analgesicusage in this small case series. The findings should be interpreted cautiously due to the small sample size and design of the case series. Controlled clinical studies are necessary.
Keywords: Photobiomodulation; primary dysmenorrhea; menstrual pain; non-pharmacological therapy; near-infrared therapy, LED therapy; menstrual cramps; low-level light therapy; red light therapy
Abbreviations List
- AI – Artificial Intelligence
- ATP – Adenosine Triphosphate
- CARE – CAse REport Guidelines
- COX-2 – Cyclooxygenase-2
- EC – Ethics Committee
- EQ-5D – EuroQol 5-Dimension Quality-of-Life Instrument
- GCP – Good ClinicalPractice
- IFU – Instructions for Use
- IRB – Institutional ReviewBoard
- ISO – International Organization for Standardization
- LED – Light-Emitting Diode
- NIR – Near-Infrared
- NO – Nitric Oxide
- NSAID – Non-Steroidal Anti-Inflammatory Drug
- PBM – Photobiomodulation
- PGF2α – Prostaglandin F2-alpha
- PGIC – Patient GlobalImpression of Change
- PROMIS – Patient-Reported OutcomesMeasurement Information System
- ROS – Reactive OxygenSpecies
- VAS – VisualAnalog Scale
AI-Assistance Statement
Artificial intelligence tools were used to support language refinement, organization, formatting, and preparation of parts of this manuscript. All scientific concepts, clinical interpretations, study design decisions, results, and conclusions are entirely the authors’ original work. The authors reviewed, verified, and approved all content generated with AI assistance to ensureaccuracy and integrity.
Introduction
Photobiomodulation (PBM) therapy utilizes low-intensity visible red (≈635 nm) and near-infrared (≈880 nm) light to activatecytochrome c oxidasewithin the mitochondrial respiratory chain, thereby enhancing ATP synthesis, augmenting microvascular circulation and reducing inflammation [1]. These effects help reduce inflammatory mediators involved in generating pain.
Although NSAIDs and hormonal therapy are commonly used to treat menstrual pain,primary dysmenorrhea still affects 45–95% of women and results in substantial disability, missed work, and economic burden [2–4]. This wide prevalence range reflects differences across populations and diagnostic criteria. Pain and discomfort typically commence 24–48 hours prior to menstruation and endure for as long as 72 hours.
Excessive production of prostaglandin F2α (PGF2α) increases uterine tone, contractions, and ischemia, which results in cramping pain, nausea and impaired ability to function [3–5]. Common risk factors include younger age, not having children, heavy flow, and a family history [4,5]. The most common treatmentis NSAIDs with hormonal contraceptives used for more severe cases [2–6]. Heat therapy, exercise, acupuncture, TENS, and nutritional supplements are common non-medicinal treatments [5–7].
This case series reviews the clinical utilization of a dual- wavelength PBM device (PBM FEM belt) for dysmenorrhea management and a suggested pilot controlled clinical trial.
Device Description
The PBM FEM belt (Figure 1) is a wellness device designedspecifically for the female pelvic anatomy. It uses a dual- wavelength LED configuration: 33% red (635 nm) LEDs for superficial tissues and 66% near-infrared (880 nm) LEDs for deeper tissues (≈1–8 mm). The PBM FEM belt’s averageirradiance is 85.13 mW/cm² for the belt.

Figure 1. PBM FEM belt with LED pad, battery, sleeve,and power cable.
The PBM FEM belt includes: the ergonomic dual-wavelength LED treatment pad (635 nm and 880 nm), built-in battery module,control interface, and cable system.
The PBM FEM device is designed to work below 42°C, whichensures non-thermal operation and should not irritate the skin. By activating cytochrome c oxidase, the FEM belt boosts ATP production, facilitates nitric oxide release, and reduces inflammatory mediators [1].
A 15-minute treatment session providesapproximately 75 J/cm² of energy.The belt is designed to be worn against clean,bare skin for best results (Figure 2). It should not be used on areas with possible or current infection.
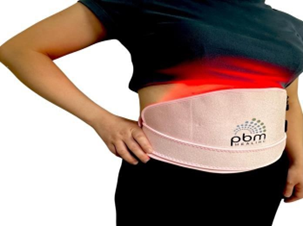
Figure 2. Application of the PBM FEM belt over the lower abdomen.
The PBM FEM belt is shown with the ergonomic dual-wavelength LED treatment pad over the lower abdominalregion. During a 15- minute session, the device providesred and near-infrared light to target the pelvic tissuesthat are relatedto primary dysmenorrhea.
Table 1. FEM Wavelength Specifications and Applications Methods Study Design
| Wavelength | Penetration Depth | Applications | Mechanism |
| 635 nm 1/3 | 1–2 mm | Decrease inflammation; superficial tissue healing | Stimulate collagen; Activate fibroblasts, superficial anti- inflammation |
| 880 nm 2/3 | 6–8 mm | Menstrual pain;deeper musculoskeletal tissues | Nitric oxiderelease; improved microcirculation, deeper ATP enhancement |
A retrospective observational case series was completed using structured, self-completed case report forms with no exclusions.
Data Collection Procedures
Standardized Visual Analog Scale (VAS 0–10) handouts wereprovided to participants before device use, with instructions to recordtheir baseline pain at the onsetof symptoms. Participants then repeated VAS scoring after completing the treatment period for that menstrual cycle.These written self-completed forms also captured medication use, duration of relief, satisfaction (1–5), and willingness to recommend the device (1–10). All VAS forms were collected at the end of the review period and entered into a secure electronic spreadsheet for analysis. This approach ensured uniform data capture and helped minimize recall bias in a retrospective series.
Participants
Inclusion criteria: women aged 18–40 years with clinically confirmed primary dysmenorrhea (no pelvic pathology via patient reported history), baseline VAS ≥7, and no concurrent experimental therapy.All participants providedwritten informed consent.
This retrospective series included all three eligible women who met the inclusion criteria during the observation period. There were no cases which were removed, excluded, or selectively reviewed. Reporting all available cases reduces the risk of selection bias, which is particularly important in very small case series.
Intervention
Participants used thePBM FEM belt intermittently for 15-minute sessions, 2 to 4 times daily, on painful days of the menstrual cycle (typically 4–6 days). The belt was placed directly to the lower abdomen. At 75 J/cm² per 15-minute session, daily cumulative fluence dose was approximately 150–300 J/cm², depending on use frequency.
Outcomes
Primary outcome: Change in VAS pain score. Secondaryoutcomes: Change in pain levels, medication usage, satisfaction (1–5), and recommendation score (1–10). The data were summarized descriptively.
Results
All three women reported baseline VAS scores of 10, indicating severe menstrual pain. After one day of use of the FEM belt, average VAS decreased to 1 (≈90% reduction). Mean post- treatment VAS was 1.0 ± 0.0 (baseline 10 ± 0.0). Two womenexperienced immediate relief; the third reported same-day improvement. All three reduced or discontinued analgesic medications. Satisfaction was uniformly 5/5, and recommendation scores were 10/10. Statistical analysis was not powered for inferential stats as the N was only 3.
There were no reported adverse effects from usage of the PBMFEM device.
Table 2. Individual Case Data
| Case | Age | Baseline VAS | After VAS | Response/Pain | Medication usage | Satisfaction | Recommendation |
| YK | 49 | 10 | 1 | Immediate; lasted entirecycle | Reduced to none | 5 | 10 |
| H | 45 | 10 | 1 | Immediate; lasted entirecycle | Eliminated | 5 | 10 |
| SO | 35 | 10 | 1 | Same-day; lasted entirecycle | Reduced significantly | 5 | 10 |
Discussion
PBM reduces dysmenorrhea symptoms via stimulation of mitochondria and enhancing microvascular perfusion. The 635/880-nm wavelengths activate cytochrome c oxidase, enhance ATP production, generate short-lived ROS signaling, and promote NO release. These effects align with the pathophysiology of primary dysmenorrhea, which involves elevated prostaglandins, COX-2 activation, ischemia, and uterine hypercontractility [1,3,7].Non-pharmacological approaches such as heat therapy, acupuncture, and TENS have mixed evidencein dysmenorrhea, and their effectiveness often depends on how often they are used and how well the person tolerates them [5–7]. PBM differs from thermotherapy andneuromodulatory methods because its primary mechanism of action occurs at the mitochondrial level. This biochemical pathway may elucidate the swift onset of relief noted in our cases.
PBM Comparison with Other Non-Pharmacological Options
Heat therapy relies on surface heating to induce muscle relaxation, whereas TENS modulates nociception through peripheral nerve electrical stimulation. PBM, in contrast, acts primarily at the mitochondrial level. Red (635 nm) and near- infrared (880 nm) wavelengths stimulate cytochrome c oxidase, enhancing ATP production, nitric oxide release, and transient ROS signaling. Thesepathways produce downstream anti-inflammatory, vasodilatory, and analgesic effects that differ fundamentally from those achieved through external heat or neuromodulation [1,6,7]. This mitochondrial mechanism may explain the rapid relief observed in our cases,as PBM can directly modulate prostaglandin-driven dysmenorrhea pathways rather than treating symptoms indirectly.
All three participants experienced a swift and clinically meaningful reduction in their pain levels, potentially due to the modulatory effects of PBM. The cordless FEM belt makes treatment easy and convenient and allows the user to direct its placement and effect.
In all three cases, VAS pain decreased from 10 to 1 after one day of use. In two of the cases, pain relief was immediate, and in the third case, relief happened later on the same day. Everyone felt better throughout their entire menstrual cycle, and all decreased pain medication usage.
Multiple PBM studies have been shown to have pain-relievingand anti-inflammatory effects [1,6,7]and the reported patient data from this series aligns with the PBM pain-relief literature. Beyond gynecologic indications, PBM has been evaluated extensively for musculoskeletal pain. Randomized, placebo- controlled trials and meta-analyses in neck and other musculoskeletal disorders have shown that appropriately dosed low-level laser/PBM can produce clinically meaningful reductions in pain that persist for weeks to months after treatment. In a landmark meta-analysis of neck pain, PBM reduced pain bynearly 20 mm on a 100-mm VAS and benefits were maintained up to 22 weeks in chronic neck painpopulations [10]. Likewise, a systematic review and meta- analysis of musculoskeletal disorders reported that PBM significantly reduced pain intensity in adults when World Association for Laser Therapy (WALT) dosing recommendations were followed [11]. Additional reviews in chronic pain populations support an analgesic signal across diverse conditions when PBM parameters are optimized[12]. These data suggest that the VAS reductions observed in our primary dysmenorrhea cases may be consistent with PBM’s broader analgesic profile in pain management [10-12].
Limitations: Future studies must incorporate randomized, sham-controlled trials featuring objectivepain evaluations and extended follow-up to mitigate issues related to small sample sizes, absence of controls, placebo effects, retrospective data, and potential reporting bias. Potential sources of bias include the authors’ affiliation with the device manufacturer, retrospective data collection, and the absence of externalassessment or monitoring. To reduce bias, standardized case report forms were utilized. All participants gave written consent for data usage, and no incentives or compensationwere offered for participation. Additionally, because all three participants achieved near-identical outcomes, the risk of overestimation due to regression to the mean or expectancy effects cannot be fully excluded.
We propose a pilot multicenter randomized trial (N=100; 1:1 sham: active), assessing VAS reduction over three menstrual cycles, medication use, and quality-of-life metrics (PROMIS/EQ-5D, PGIC). The proposedtrial would use change in VAS pain score duringthe first 48 hours of menstruation as the primarymeasure. Also assessedwould be analgesicusage, PGIC, and quality-of-life measures (PROMIS/EQ-5D). The sample size of N = 100 (50 active, 50 sham) provides >80% power to detect a clinically meaningful 2-pointdifference in VAS pain reduction, assuming a standard deviation of 2.5 and α = 0.05.
The randomized trial will utilize a sham device identical in size, weight, appearance, LED layout, and user interface to maintain blinding. The sham device will includeinactive LEDs that do not emit therapeutic wavelengths. Participant, investigator, and assessor blinding will be maintained.
Given the worldwide prevalence of primary dysmenorrhea—affecting an estimated 45–95% of menstruating women—and its status as a leading cause of adolescent school absenteeism and reducedworkplace productivity, the cumulative economicburden is substantial at both individual and societal levels [2–
4]. Guidelines and population-based reviews emphasize not only the high use of NSAIDs and hormonal therapybut also the indirect costs associated with lost days of study and work, presenteeism, and impairedquality of life [2–4]. In this context,drug-free, self-managed options such as PBM devices may support women’s health equity by improving access to effective pain relief without ongoing medication costs or frequent healthcare visits, particularly for individuals who cannot tolerate or prefer to avoid pharmacologic therapies.
Conclusion
In this small, three-case series, the PBM FEM belt produced rapid and substantial non-pharmacological pain relief in all 3 women with primary dysmenorrhea. These findings areencouraging; however, they are only preliminary due to the very small sample size and absence of a control group. These observations are therefore considered hypothesis-generating. We note that a fully designed,adequately powered randomized controlled trial is required to determine the true effectiveness and generalizability of this PBM approach.
References
- Hamblin MR. Mechanisms and applications of the anti- inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi:10.3934/biophy.2017.3.337.
Publisher | Google Scholor - Burnett M, Lemyre M. No. 345 – Primary Dysmenorrhea Consensus Guideline. J Obstet Gynaecol Can. 2017;39(7):585–595. doi:10.1016/j.jogc.2016.12.023.
Publisher | Google Scholor - American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet Gynecol. 2018;132(6):e249–e258. doi:10.1097/AOG.0000000000002978.
Publisher | Google Scholor - Ferries-Rowe E, Corey E, Archer JS. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet Gynecol. 2020;136(5):1047– 1058. doi:10.1097/AOG.0000000000004096.
Publisher | Google Scholor - Gindaba BG, Sebu LD, Gindaba EZ, et al. Management practices of primary dysmenorrhea among female high school students in Nekemte town, East Wallaga Zone, Western Oromia, Ethiopia: a cross-sectional study. BMC Womens Health. 2025;25:194. doi:10.1186/s12905-025- 03732-0.
Publisher | Google Scholor - Itani R, Soubra L, Karout S, Rahme D, Karout L, Khojah HMJ. Primary dysmenorrhea: pathophysiology, diagnosis, and treatment updates. Korean J Fam Med. 2022;43(2):101–108. doi:10.4082/kjfm.21.0103.
Publisher | Google Scholor - Nagy H, Carlson K, Khan MAB. Dysmenorrhea. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025–. Updated 2023 Nov 12.
Publisher | Google Scholor - Armour M, Parry K, Manohar N, Holmes K, Smith CA. Dysmenorrhea: a narrative review of therapeutic options. J Pain Res. 2024;17:2657–2672. doi:10.2147/JPR.S464103.
Publisher | Google Scholor - PBM Healing International. PBM FEM Belt: Instructions for Use (IFU). Hong Kong: PBM Healing International; 2024.
Publisher | Google Scholor - Chow RT, Johnson MI, Lopes-Martins RAB, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment-controlled trials. Lancet. 2009;374(9705):1897–1908. doi:10.1016/S0140- 6736(09)61522-1.
Publisher | Google Scholor - Kingsley JD. Low-level laser therapy as a treatment for chronic pain: a systematic review. Lasers Med Sci. 2014;29(2):615–624.
Publisher | Google Scholor
 Alcrut
Alcrut